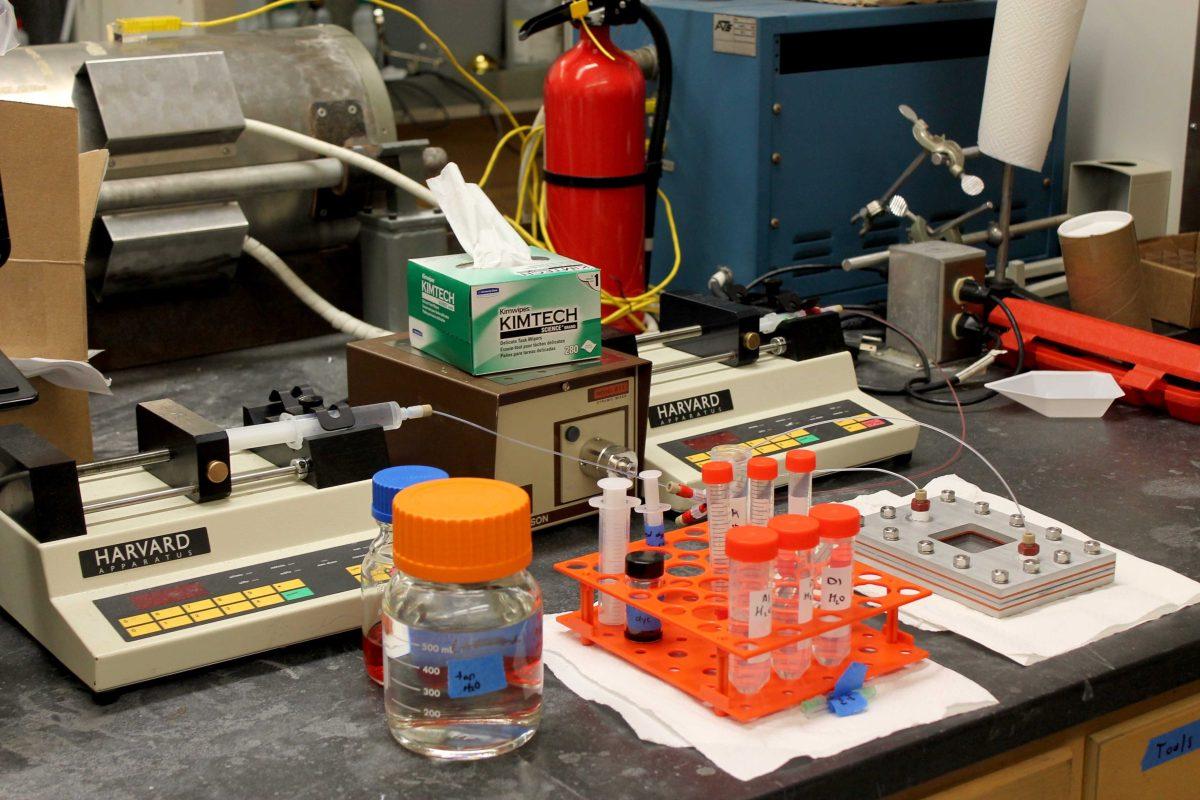
According to the World Health Organization, approximately 663 million people lack access to improved water worldwide. Kevin McPeak wants to change that.
McPeak, a chemical engineering assistant professor, is developing a method to successfully disinfect water using visible light, metal nanoparticles and oxygen. When complete, McPeak said the innovation could be used to combat water related illnesses in developing countries.
The research involves combining a semiconductor such as titanium dioxide or zinc oxide with engineered metal nanoparticles. The metal nanoparticles have a greater absorption capacity and can extend energy absorption into the visible light spectrum, he said.
Semiconductors in current disinfection systems can only absorb ultraviolet light, which constitutes only 5 percent of the electromagnetic spectrum, McPeak said. By expanding the potential absorption spectrum to include visible light, the rate of disinfection will be noticeably increased.
When dissolved oxygen in the water reacts with the catalyst particles
highly reactive oxygen species (ROS) are produced. These ROS are able
to oxidize carbon-based pathogens such as bacteria and viruses, into
carbon dioxide, neutralizing the threat.
The challenge is engineering the semiconductor-nanoparticle pairing that will produce the most successful form of reactive oxygen. Certain reactive oxygen species can combine with sinks, such as anions or sulfates, and convert back into traditional oxygen before reaching the pathogen, destroying the system’s efficacy, he said.
Chemical engineering graduate student Daniel Willis said engineering the optimum semiconductor-nanoparticle complex is the team’s primary focus at this stage. Willis said he and other researchers spend eight to 10 hours a day prepping the samples for testing in the lab’s microreactor.
The microreactor allows the research team to test the complexes in a controlled and observable environment, McPeak said. After tests in the microreactor, adjustments to the nanoparticle structures and arrangements are made to increase the complex’s efficacy, Willis said.
Despite the intensive testing process, Willis said the opportunity to learn and help others is the greatest reward.
“I was working in a microbrewery in Texas and that was fantastic … but at the end of the day think about what you contribute to the world at large,” Willis said. “With something like this you can come in with full confidence and say, ‘yes, I actually am improving the world.’ It’s nice to be a gear in a cog that’s advancing and progressing strong human causes.”
McPeak said the goal is to run initial disinfection testing using bacteriophage MS2, a virus that mimics Hepatitis and the Adenovirus, by the end of the summer. The team hopes to have a testable commercial prototype within nine months to a year, he said.
The prototype will most likely resemble a bottle, and would be filled with glass sand or a polymer coated in the semiconductor-nanoparticle complexes. The jar would then be filled with water and oxygen, shaken and left in the sun to work its magic, he said.
McPeak said the finished product’s simplicity is the key to widespread distribution.
“If we can lower the barrier of entry to obtaining clean water in the developing world, I think we could have a major social impact with this technology,” McPeak said. “It is a staggering number to think in 2016 that a billion people don’t have access to clean water, but that’s reality.”
McPeak isn’t the only person concerned about the world’s clean water supply.
BASF, a multi-national chemicals manufacturing corporation, selected McPeak and his team as the first researchers in residence for the $1 million BASF Sustainable Living Lab in the redesigned Patrick F. Taylor Hall, said BASF Southeast Regional Communications Hub’s external communications director Blythe Lamonica.
Lamonica said sustainable practices are a cornerstone of BASF’s business culture and the company aims to leverage the work of innovative researchers pursuing sustainable solutions to global issues. Clean water availability is a main focus for the company, and McPeak’s scaleable solution spoke to the company, she said.
McPeak and his team will occupy the lab for two years. In addition to being a functioning lab, the work space’s interactive television screens and tablet features also double as an outreach hub to educate students and the public about BASF’s sustainability mission and the progress of McPeak’s research, Lamonica said.
Having a dedicated workspace will be a big moral boost for the team, McPeak said.
“Oftentimes, the grand idea is easily lost when you’re in the trenches and things aren’t working out,” McPeak said.
Having a dedicated space signals to both the researchers and the public that the research is significant, and acts as an everyday motivation to continue pushing forward, he said.
A previous version of this article incorrectly stated: “The disinfection occurs when oxygen is introduced to the system. The oxygen reacts with the semiconductor-nanoparticle complex to produce a reactive oxygen species, such as hydrogen peroxide, McPeak said. The reactive oxygen species will then convert the carbon based bacteria and viruses into carbon dioxide, neutralizing the threat, he said.”
Chemical engineering graduate student Daniel Willis and other researchers are spearheading an effort to develop a portable water purification system.